Medtronic PLC NYSE: MDT, a member of the medical sector and the healthcare industry, received U.S. FDA approval for its implantable medical device called the Percept PC Deep Brain Stimulation (DBS) system on January 8. This system includes the PC neurostimulator, BrainSense technology and SenSight directional leads.
It treats movement disorders like debilitating tremors experienced by patients with Parkinson's disease, epilepsy and dystonia, which impact more than 11 million people in the United States.
Implantable devices
The notion of human implantable devices conjures up images of the Terminator and a cyberpunk world controlled by cyborgs. However, the reality is that implantable devices have been around for over 50 years. Experts invented cardiac pacemakers in the late 1950s, cochlear implants in the 1960s and implantable cardioverter defibrillators (ICDs) in the 1980s for cardiac arrhythmia detection. Implantable insulin pumps became available in the 1990s.
Deep brain stimulation
The science of deep brain stimulation (DBS) has been around since the 1990s and FDA approved since 2002. While much of the brain is still a mystery, scientists have discovered that sending electronic pulses to certain brain parts can directly impact various physical symptoms. It's the belief that the brain is misfiring the electric impulses, an electrical stimulating abnormality, leading to physical symptoms like tremors, slowness and stiffness.
DBS seeks to correct the situation by delivering controlled and adjustable electrical stimulation into the electrical pathway firing incorrectly to stabilize the symptoms. DBS systems consist of an implantable pulse generator (IPG) under the skin on the chest that sends electric pulses through the electrodes implanted into specific areas of the brain.
Percept DBS system
The Percept PC featuring BrainSense technology gives real-time brain data, which enables data-driven and personalized therapy and the ability to make adjustments as needed. SenSight directional leads enable precision stimulation to specific areas of the brain directly.
The implant process is a minimally invasive surgery procedure where surgical leads get put in the brain. Doctors place the neurostimulator near the chest or abdomen, like a pacemaker. Doctors use the external programming device to adjust the settings for stimulation to optimize a patient's therapy.
Improve symptoms, not cure the disease
The system helps to improve the symptoms significantly, but it's not a cure. For Parkinson's disease patients, it can significantly improve bradykinesia (slowness of movement), rigidity and tremors. For patients with epilepsy, DBS can help reduce the severity and frequency of seizures. For dystonia, it can provide some relief from abnormal postures and involuntary muscle contractions. It's an effective treatment for essential tremors in patients without movement relief from other therapies.
Steady earnings beats
Medtronic reported fiscal Q2 2024 EPS of $1.25 per share, beating analyst expectations by seven cents on November 21, 2023. Revenues climbed 5.3% YoY to $7.98 billion, beating $7.93 billion consensus analyst estimates. Medtronic has been proactively cutting costs, targeting $1 billion in savings by 2025.
Cardiovascular products are the primary revenue driver.
The company derives a significant portion of revenues (36.6%) from its cardiovascular medical devices like pacemakers, catheters, stents, heart valve replacement, electrosurgical, infusion therapy, revascularization, and cardiopulmonary products. Cardiovascular products are expected to have a 7.6% CAGR through 2032. They are the leading global manufacturer of implantable cardiac devices, competing with Abbott Laboratories Inc. NYSE: ABT, which became a major player upon its complementary acquisition of St. Johns Medical and its portfolio of stents and pacemakers.
Mixed guidance
Medtronic raised forecasts for the fiscal full-year 2024 EPS to $5.13 to $5.19, up from the previous forecast of $5.08 to $5.12 versus $5.12 consensus analyst estimates. Fiscal full-year 2024 revenues are expected to grow 2.6% or $32.04 billion, falling short of $32.17 billion consensus estimates. Full-year organic growth guidance was raised to 4.75%, up from 4.5%.
Medtronic analyst ratings and price targets are at MarketBeat. You can find Medtronic peers and competitor stocks with the MarketBeat stock screener. Medtronic is a Dividend Aristocrat paying a 3.17% annual percentage yield.
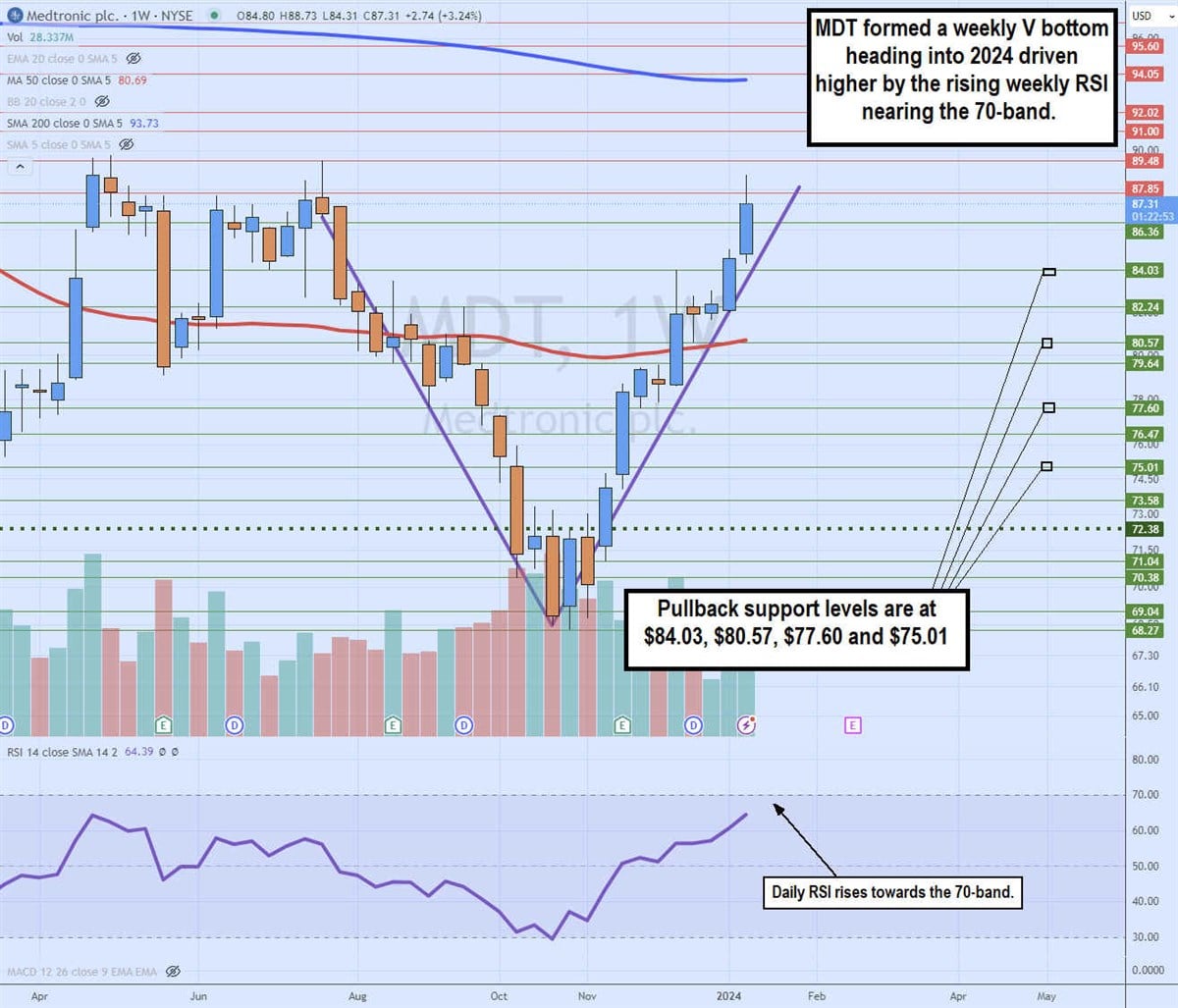 Weekly V bottom
Weekly V bottom
The weekly candlestick chart for MDT illustrates the classic V bottom. MDT peaked at $89.48 in July 2023 and collapsed to a low of $68.27 by October 2023.
MDT formed a low market structure (MSL) on the bounce and breakout through the $72.38 trigger on November 13, 2023. Shares proceeded to rally up through the weekly 50-period moving average at $80.69. The upside momentum continued, driven by the rising weekly relative strength index (RSI) oscillator climbing towards the 70-band. MDT peaked at $88.73, looking for a gap fill and V completion at $89.48. Pullback supports are at $84.03, $80.57, $77.60 and $75.01.
Before you consider Medtronic, you'll want to hear this.
MarketBeat keeps track of Wall Street's top-rated and best performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat has identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market catches on... and Medtronic wasn't on the list.
While Medtronic currently has a Moderate Buy rating among analysts, top-rated analysts believe these five stocks are better buys.
View The Five Stocks Here
Discover the top 7 AI stocks to invest in right now. This exclusive report highlights the companies leading the AI revolution and shaping the future of technology in 2025.
Get This Free Report
Like this article? Share it with a colleague.
Link copied to clipboard.