Clinical weight-loss medications have been making headlines. GLP-1 drugs like Novo Nordisk A/S NYSE: NVO owned Ozempic and Wegovy and Eli Lilly and Co. NYSE: LLY owned Mounjaro and Zepbound continue to see demand outstrip supply.
Celebrities like Sharon Osbourne lost 42 pounds, and Oprah Winfrey lost 60 pounds. The mainstream has jumped on board with these weight-loss medications. However, the markets are always looking for new medicines from biotechs in the medical sector that may lessen some side effects of nausea, vomiting and gastrointestinal issues.
Here are two companies with weight loss medications in their pipeline.
Viking Therapeutics Inc.
Viking Therapeutics Inc. NASDAQ: VKTX is a clinical-stage biotech focused on developing treatments for metabolic and endocrine disorders. The company's top candidate is VK2735, a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). Rather than target one hormone, this medication targets two agonists in GLP-1 and GIP, similar to Mounjaro and Zepbound. Viking is working on both an oral formulation and an injection.
Phase 1 trials
The Phase 1 trial performed two studies for adults with a BMI of 30 or higher. It tested a single-ascending dose (SAD), which is a single dose, to observe safety and tolerability, which all participants continued with minor tolerable side effects. No participants dropped out due to adverse effects.
The multiple ascending dose (MAD) study indicated the average body weight reduction from baseline up to 7.8% after 21 days. All MAD cohorts saw a reduction in mean body weight. Mean bodyweight relative to placebo was up to 6%. Safety and tolerability were the main goals, and VK2735 achieved them. It also showed a reduction in positive trends in lipids and liver enzymes. It opened the door to move onto Phase 2 trials, where doses escalated.
Phase 2 trials
The Phase 2 VENTURE trials completed enrollment in October 2023 with 176 adult participants. The 13-week closing window assesses the weekly injections at multiple doses compared to placebo. The primary endpoint from baseline to week 13 will be the percentage change in body weight compared to the placebo. Secondary endpoints further evaluate safety, tolerability, and efficacy measures, including changes in metabolic markers, body composition, and appetite control. The data should present in the first half of 2024.
Check out the sector heatmap on MarketBeat.
VK2809 and cash position
Viking's most advanced pipeline treatment is VK2809 for treating non-alcoholic steatohepatitis (NASH). It is a condition where excess fat builds up in the liver, causing inflammation and damage to liver cells. NASH is not caused by excessive alcohol consumption, which is an alcoholic form of fatty liver disease. The drug attempts to reduce the fat in the liver, which also relates to VK2735, which has shown improvement in liver enzymes.
Altimmune Inc. NASDAQ: ALT has a GLP-1 candidate, pemvidutide, intended to treat NASH. It has shown an average weight loss of 15.6% after 48 weeks and a significant reduction in liver fats, with some patients even normalizing liver fat. Viking Therapeutics has $376 million in cash and cash equivalents to keep operations ongoing for years.
Viking Therapeutics analyst ratings and price targets are at MarketBeat. You can find Viking Therapeutics peers and competitor stocks on the MarketBeat stock screener.
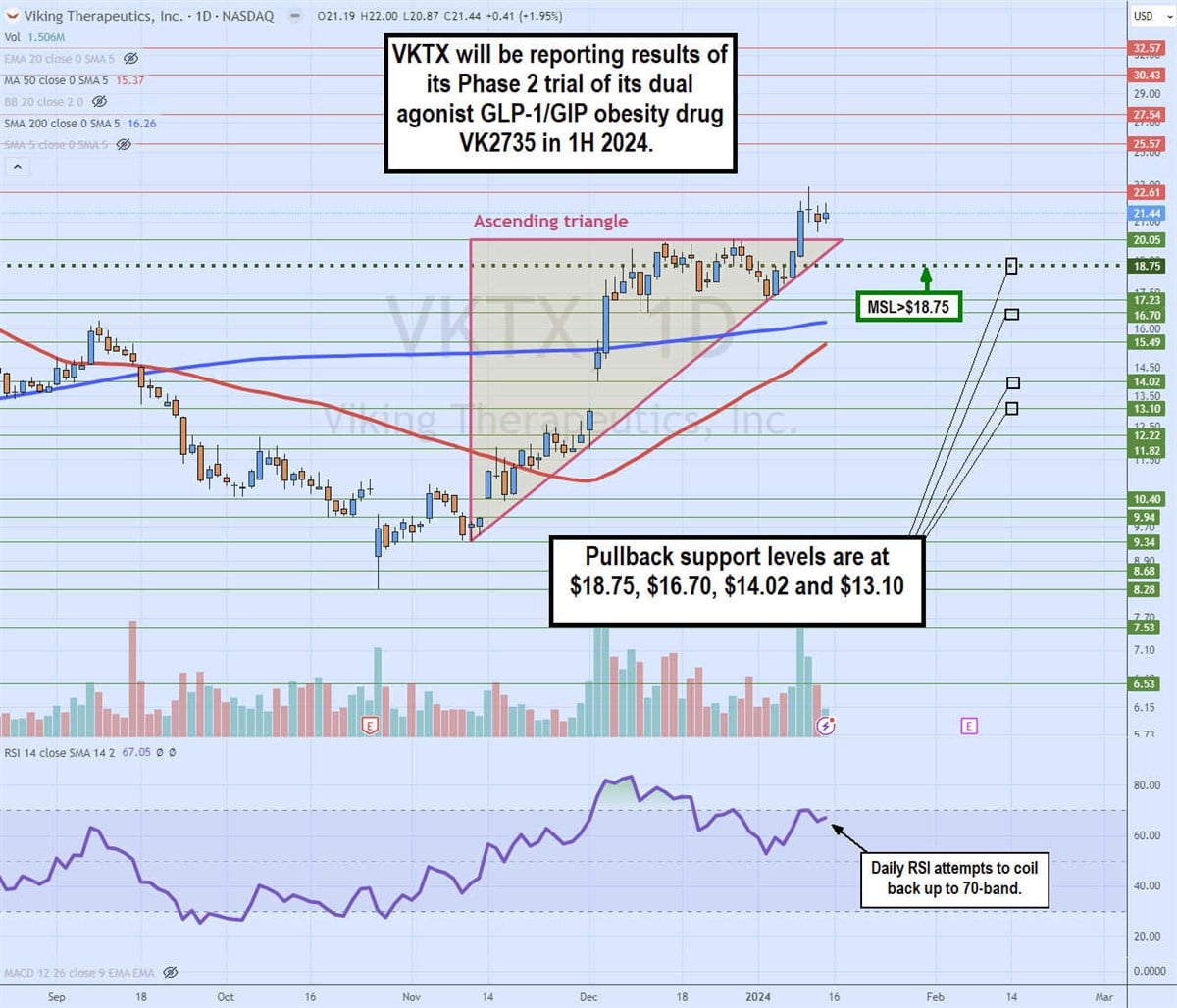
Daily ascending triangle
The daily candlestick chart for VKTX illustrates a daily ascending triangle pattern. The ascending (rising) trendline commenced at $9.34 on November 9, 2023, indicating higher lows on pullbacks. VKTX staged a rally through the daily 200-period moving average (MA), currently at $16.26, to reject at the flop-top upper trendline at $20.05.
VKTX triggered a market structure low (MSL) breakout at $18.75 to break through the flat-top upper trendline on January 9, 2024, peaking at $22.91. The daily relative strength index (RSI) is attempting to coil back up through the 70-band. Pullback support levels are at $18.75, $16.70, $14.02 and $13.10.
Rhythm Pharmaceuticals Inc.
Rhythm Pharmaceuticals Inc. NASDAQ: RYTM is a commercial-stage biotech specializing in developing treatments for rare genetic diseases of obesity. They focus on treating kids and adults living with hyperphagia and severe obesity caused by melanocortin-4 receptor (MC4R) pathway diseases.
Hyperphagia
Hyperphagia, also called polyphagia, is an abnormally strong, compulsive, and persistent sensation of hunger that leads to overeating and obesity. People with hyperphagia can feel extreme hunger even after finishing a large meal and end up having to gorge on food constantly to satisfy this uncontrollable compulsion to eat.
Bardet-Biedl Syndrome
Hyperphagia is a prominent symptom of genetic diseases like Bardet-Biedl syndrome (BBS), which leads to obesity, vision and kidney problems, developmental and cognitive delays, and skeletal and genital abnormalities. It's believed that disruptions in the MC4R pathway cause BBS.
IMCIVREE (Setmelanotide)
Rhythm's lead drug, IMCIVREE, is an FDA-approved MC4R agonist that stimulates the receptor to help regulate appetite and weight management for patients suffering from genetic conditions like BBS. The drug has been FDA-approved since November 2020 for rare genetic diseases of obesity.
It’s used for chronic weight management for children six and older and adults with obesity due to leptin receptor (LEPR), Proopiomelanocortin (POMC) or proprotein convertase subtilisin/kexin type (PCSK1) deficiency. IMCIVREE was designed to restore impaired MC4 receptor pathway activity. Phase 3 clinical trials resulted in 80% of participants with obesity due to POMC or PCSK1 deficiency having 10% or more weight loss, and 45.5% with LEPR deficiency achieved more than 10% weight loss after one year of treatment. The mean BMI reduction was 25.5% after a year of setmelanotide therapy.
Hypothalamic obesity
The FDA approved IMCIVREE for BBS patients in June of 2022. The results of a study of IMCIVREE in hypothalamic obesity (HO). This is a type of obesity caused by a damaged hypothalamus, a part of the brain responsible for regulating appetite and body weight.
A damaged hypothalamus can lead to weight gain. It can happen from brain injury, brain tumors and genetic mutations resulting in rapid weight gain, uncontrollable appetite, and decreased energy. There is currently no cure for it. Rhythm's HO study was promising, with all 11 patients reaching the endpoint of more than a 5% reduction in BMI after four months of Setmelanotide therapy and a mean change in hunger score of -2.7 and BMI of -17.2%.
RP501 (Retatrutide)
Currently, in Phase 2b trials, this drug targets three hormones, GLP-1, GIP, and glucagon, which are believed to influence appetite.
Driving revenues
IMCIVREE remains the only treatment for MC4R pathway-related obesity disorders approved by the FDA, which could impact over 130,000 people in the United States and the European Union. It's also been the key revenue driver for the company. Its Q3 2023 revenues surged 425% YoY to $22.5 million. Net losses were $40.9 million or 79 cents per share.
Developments
The company had more than 120 new prescriptions for IMCIVREE for BBS and received payor approval for 80 prescriptions in the quarter. Treatment is around $240,000 per patient. The company is also commencing Phase 3 clinical trials for HO with 140 patients, which is expected to report results by 1H 2025. The company ended the quarter with $299.5 million in cash and cash equivalents.
On January 4, 2024, the FDA accepted the investigation of a new drug application (IND) for RM-718, a weekly treatment designed to be more targeted and potent than Setmelanotide without causing hyperpigmentation. Human phase-1 trials are expected in 1H 2024, including a MAD study in patients with HO. Get AI-powered insights on MarketBeat.
Rhythm Pharmaceuticals analyst ratings and price targets are at MarketBeat. Rhythm Pharmaceutical's peers and competitor stocks can be found with the MarketBeat stock screener. Editas has an 18.33% short interest.
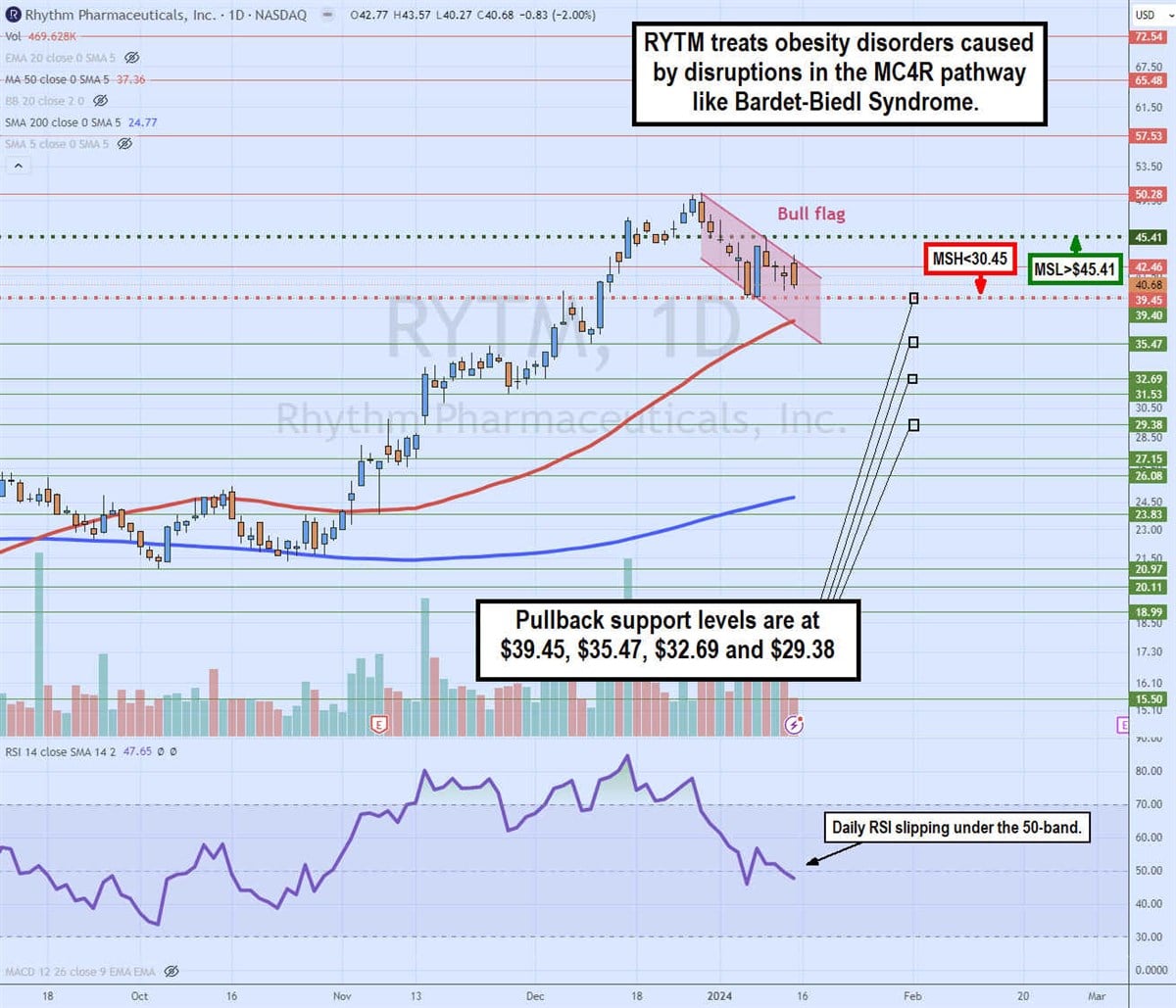
Daily bull flag and dueling market structure triggers
The daily candlestick chart on RYTM illustrates a potential bull flag pattern. It also presents an interesting case of dueling market structure signals. The daily market structure low (MSL) buy triggers a breakout through $45.41, which would also trigger the bull flag breakout.
The weekly market structure high (MSH) sell triggers a breakdown below $30.45. The bull flag commenced after peaking at $50.28 on December 28, 2023. RYTM shares pulled back in a parallel channel as the relative strength index (RSI) also fell under the overbought 70-band. The daily 50-period MA support is rising at $37.36, followed by the daily 200-period MA support at $24.77. Pullback support levels are at $39.45, %35.47, $32.69 and $29.38.
Before you consider Viking Therapeutics, you'll want to hear this.
MarketBeat keeps track of Wall Street's top-rated and best performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat has identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market catches on... and Viking Therapeutics wasn't on the list.
While Viking Therapeutics currently has a Buy rating among analysts, top-rated analysts believe these five stocks are better buys.
View The Five Stocks Here
Wondering where to start (or end) with AI stocks? These 10 simple stocks can help investors build long-term wealth as artificial intelligence continues to grow into the future.
Get This Free Report
