 In this Tuesday, Dec. 8, 2020 file photo, Dr. Rochelle Walensky, speaks during an event in Wilmington, Del., to announce President-elect Joe Biden's health care team. Walensky, 51, an infectious-diseases specialist formerly at Harvard Medical School and Massachusetts General Hospital, became CDC director on Wednesday, Jan. 20, 2021. (AP Photo/Susan Walsh)
In this Tuesday, Dec. 8, 2020 file photo, Dr. Rochelle Walensky, speaks during an event in Wilmington, Del., to announce President-elect Joe Biden's health care team. Walensky, 51, an infectious-diseases specialist formerly at Harvard Medical School and Massachusetts General Hospital, became CDC director on Wednesday, Jan. 20, 2021. (AP Photo/Susan Walsh) In this Jan. 21, 2021, photo, Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks with reporters in the James Brady Press Briefing Room at the White House in Washington. President Joe Biden is dispatching the nation’s top scientists and public health experts to regularly brief the American public about the pandemic. Beginning Jan. 27, the experts will host briefings three times a week on the state of the outbreak and efforts to control it. (AP Photo/Alex Brandon)
In this Jan. 21, 2021, photo, Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks with reporters in the James Brady Press Briefing Room at the White House in Washington. President Joe Biden is dispatching the nation’s top scientists and public health experts to regularly brief the American public about the pandemic. Beginning Jan. 27, the experts will host briefings three times a week on the state of the outbreak and efforts to control it. (AP Photo/Alex Brandon) White House press secretary Jen Psaki speaks during a press briefing at the White House, Friday, Jan. 29, 2021, in Washington. (AP Photo/Evan Vucci)
White House press secretary Jen Psaki speaks during a press briefing at the White House, Friday, Jan. 29, 2021, in Washington. (AP Photo/Evan Vucci) German Klaus Schwab, left, Founder and Executive Chairman of the World Economic Forum, WEF, listens Japanese Prime Minister Yoshihide Suga displayed in screens during a video conference at the Davos Agenda, in Cologny near Geneva, Switzerland, Friday, January 29, 2021. The Davos Agenda, from 25 to 29 January 2021, is a online edition due to the coronavirus disease (COVID-19) outbreak gather global leaders to shape the principles, policies and partnerships needed in this challenging new context. (Salvatore Di Nolfi/Keystone via AP)
German Klaus Schwab, left, Founder and Executive Chairman of the World Economic Forum, WEF, listens Japanese Prime Minister Yoshihide Suga displayed in screens during a video conference at the Davos Agenda, in Cologny near Geneva, Switzerland, Friday, January 29, 2021. The Davos Agenda, from 25 to 29 January 2021, is a online edition due to the coronavirus disease (COVID-19) outbreak gather global leaders to shape the principles, policies and partnerships needed in this challenging new context. (Salvatore Di Nolfi/Keystone via AP)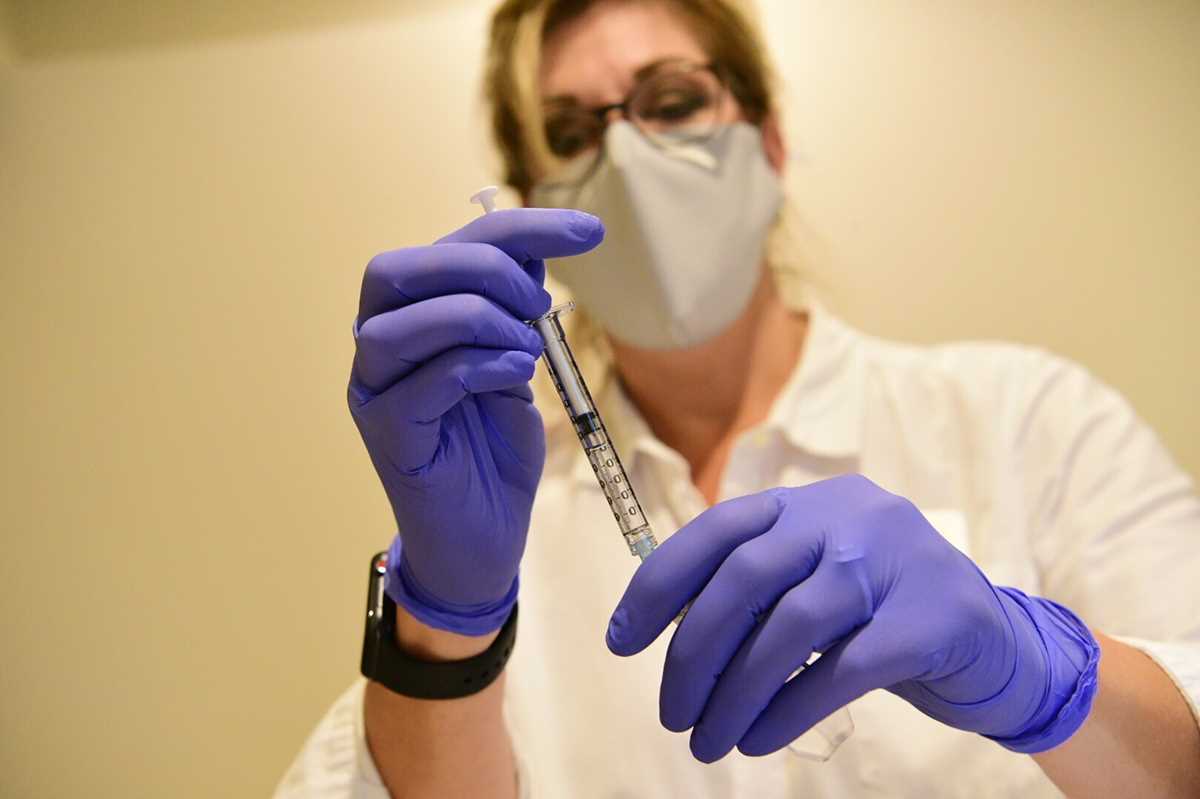 This Sept. 2020 photo provided by Johnson & Johnson shows a clinician preparing to administer investigational Janssen COVID-19 vaccine. Johnson & Johnson's long-awaited COVID-19 vaccine appears to protect against symptomatic illness with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. Johnson & Johnson said Friday, Jan. 29, 2021 that in the U.S. and seven other countries, the first single-shot vaccine appears 66% effective overall at preventing moderate to severe COVID-19. It was more protective against severe symptoms, 85%. (Johnson & Johnson via AP)
This Sept. 2020 photo provided by Johnson & Johnson shows a clinician preparing to administer investigational Janssen COVID-19 vaccine. Johnson & Johnson's long-awaited COVID-19 vaccine appears to protect against symptomatic illness with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. Johnson & Johnson said Friday, Jan. 29, 2021 that in the U.S. and seven other countries, the first single-shot vaccine appears 66% effective overall at preventing moderate to severe COVID-19. It was more protective against severe symptoms, 85%. (Johnson & Johnson via AP)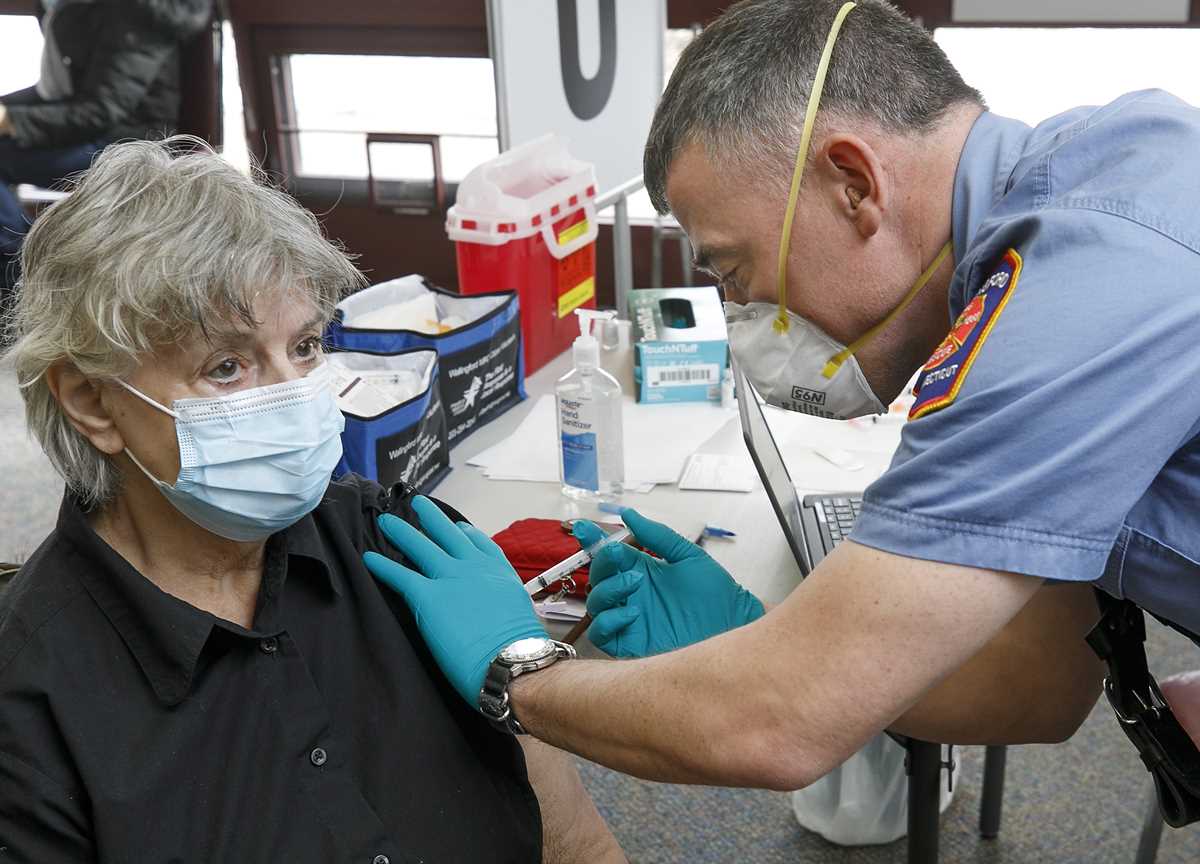 Wallingford fire department EMS Lt. Michael Krupinski administers the COVID-19 vaccine to Gail Morse, of Wallingford, Conn., at the Wallingford Senior Center, Thursday., Jan. 28, 2021. (Dave Zajac /Record-Journal via AP)
Wallingford fire department EMS Lt. Michael Krupinski administers the COVID-19 vaccine to Gail Morse, of Wallingford, Conn., at the Wallingford Senior Center, Thursday., Jan. 28, 2021. (Dave Zajac /Record-Journal via AP) In this Saturday, July 18, 2020 file photo a general view of AstraZeneca offices and the corporate logo in Cambridge, England. The European Medicines Agency is expected on Friday Jan. 29, 2021 to authorize use of the vaccine AstraZeneca developed with Oxford University. It would be the third cleared for use in the EU, after the BioNTech-Pfizer and Moderna vaccines. (AP Photo/Alastair Grant, File)
In this Saturday, July 18, 2020 file photo a general view of AstraZeneca offices and the corporate logo in Cambridge, England. The European Medicines Agency is expected on Friday Jan. 29, 2021 to authorize use of the vaccine AstraZeneca developed with Oxford University. It would be the third cleared for use in the EU, after the BioNTech-Pfizer and Moderna vaccines. (AP Photo/Alastair Grant, File)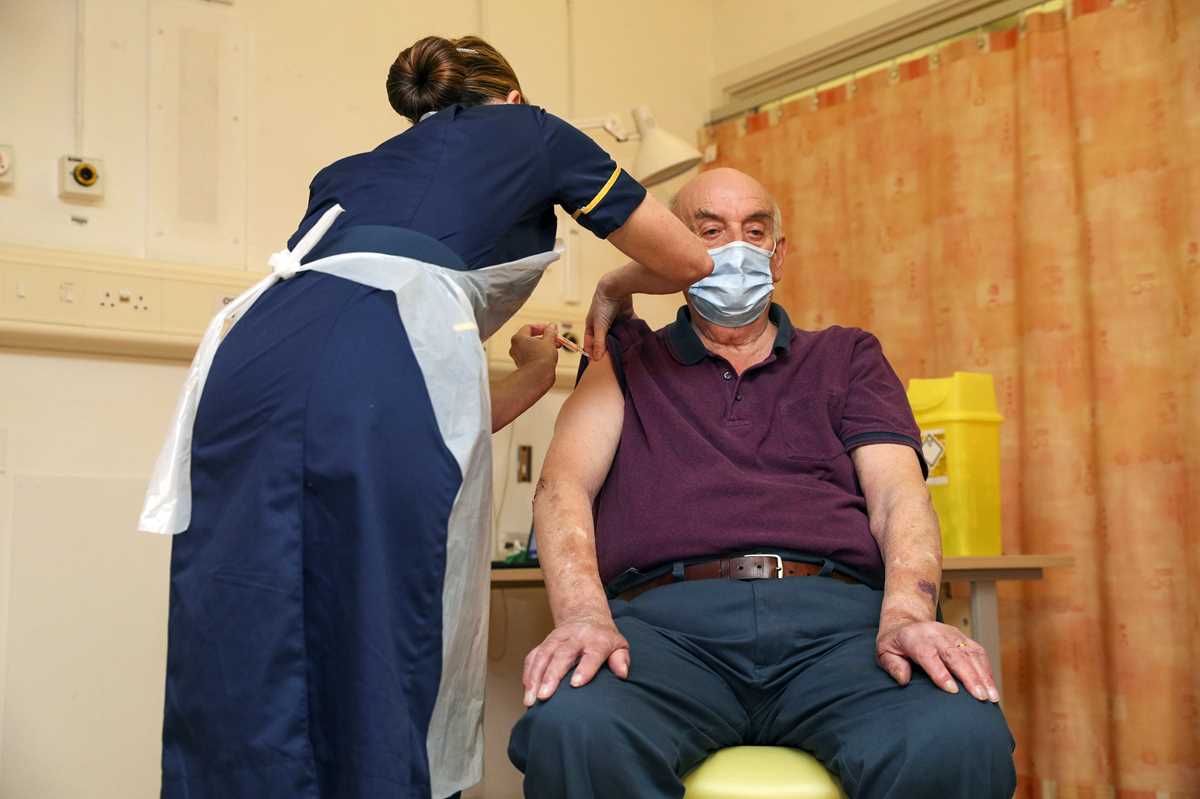 In this Monday, Jan. 4, 2021 file photo, 82-year-old Brian Pinker receives the Oxford University/AstraZeneca COVID-19 vaccine from nurse Sam Foster at the Churchill Hospital in Oxford, England. The European Medicines Agency is expected on Friday Jan. 29, 2021 to authorize use of the vaccine AstraZeneca developed with Oxford University. It would be the third cleared for use in the EU, after the BioNTech-Pfizer and Moderna vaccines. (Steve Parsons/Pool Photo via AP, File)
In this Monday, Jan. 4, 2021 file photo, 82-year-old Brian Pinker receives the Oxford University/AstraZeneca COVID-19 vaccine from nurse Sam Foster at the Churchill Hospital in Oxford, England. The European Medicines Agency is expected on Friday Jan. 29, 2021 to authorize use of the vaccine AstraZeneca developed with Oxford University. It would be the third cleared for use in the EU, after the BioNTech-Pfizer and Moderna vaccines. (Steve Parsons/Pool Photo via AP, File)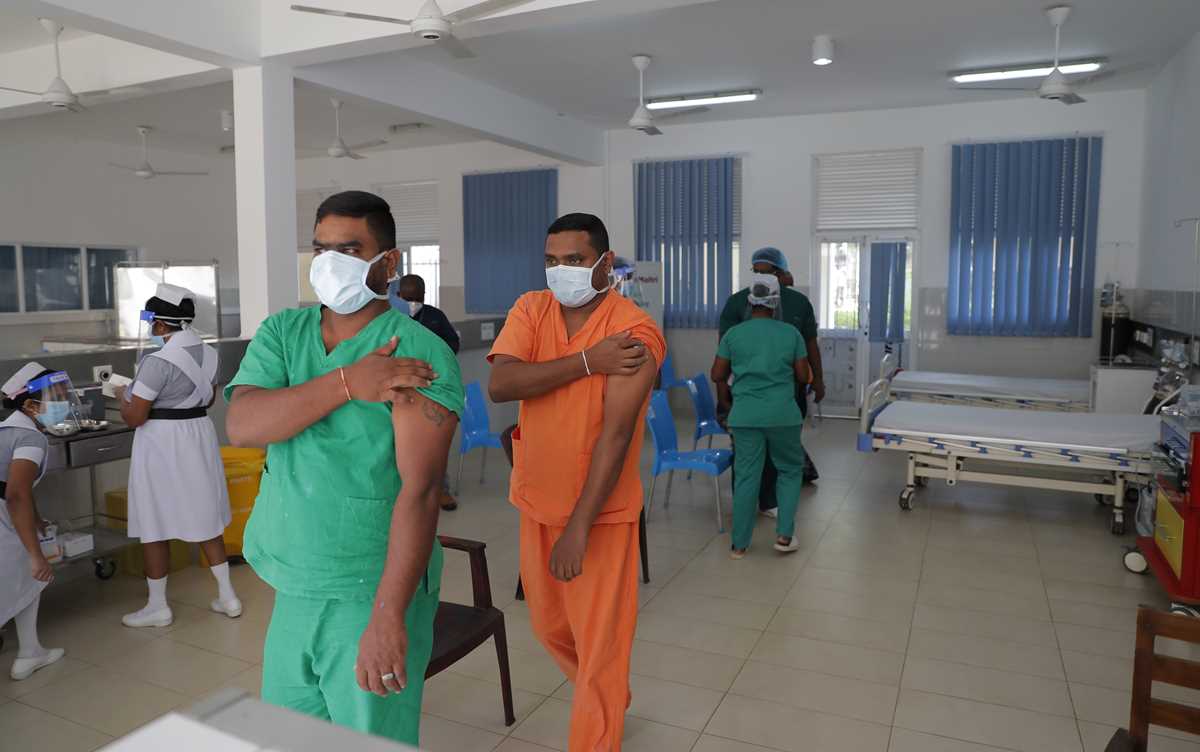 Sri Lankan health workers leave after receiving COVID-19 vaccines in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)
Sri Lankan health workers leave after receiving COVID-19 vaccines in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)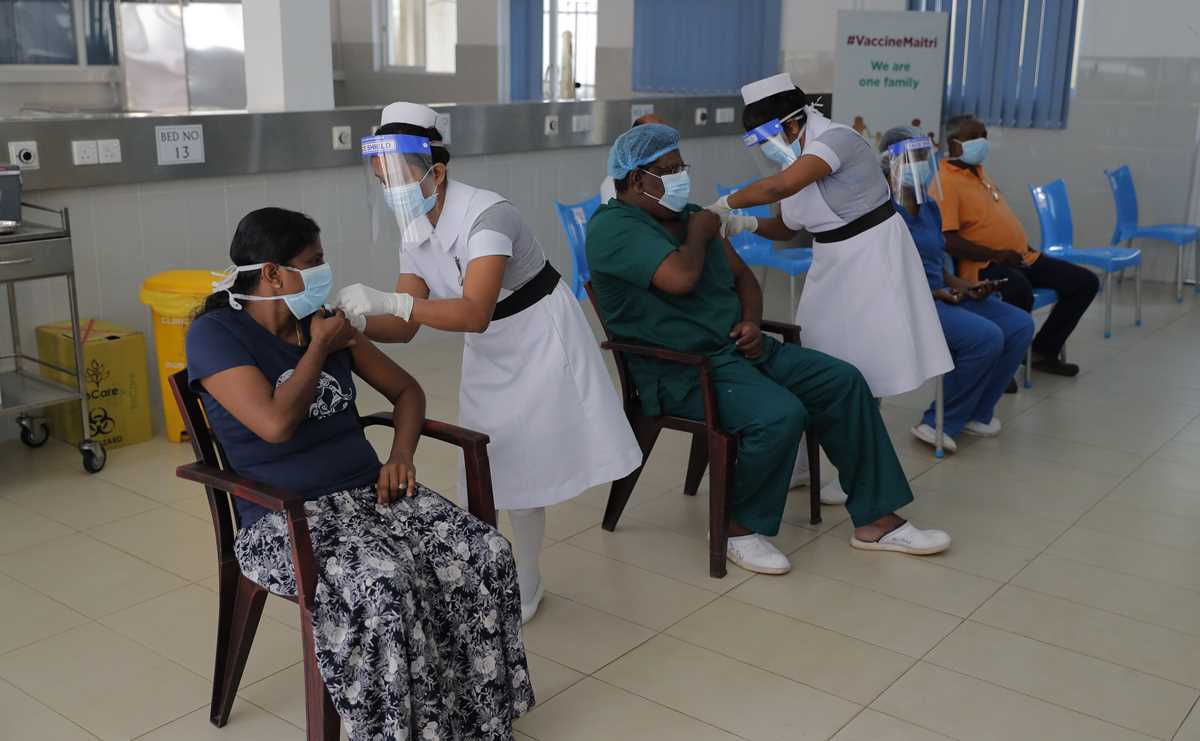 Sri Lankan nursing staff administer COVID-19 vaccines to frontline health workers in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)
Sri Lankan nursing staff administer COVID-19 vaccines to frontline health workers in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena) Sri Lankan army soldiers wait to receive COVID-19 vaccines in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)
Sri Lankan army soldiers wait to receive COVID-19 vaccines in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena) A worker gives final touches to a graffiti that tributes to Malaysian workers on the frontline against the COVID-19 pandemic outside a hospital in Kuala Lumpur, Malaysia, Friday, Jan. 29, 2021. Malaysian authorities imposed tighter restrictions on movement to try to halt the spread of the coronavirus. (AP Photo/Vincent Thian)
A worker gives final touches to a graffiti that tributes to Malaysian workers on the frontline against the COVID-19 pandemic outside a hospital in Kuala Lumpur, Malaysia, Friday, Jan. 29, 2021. Malaysian authorities imposed tighter restrictions on movement to try to halt the spread of the coronavirus. (AP Photo/Vincent Thian) In this July 11, 2020, file photo, party members have their temperature checked and sanitize their hands as a precaution against the coronavirus at the national congress of the ruling Chama cha Mapinduzi (CCM) party in Dodoma, Tanzania. Tanzania's President John Magufuli openly expressed doubt about COVID-19 vaccines and accused people who were vaccinated outside the East African nation of bringing new infections into the country. (AP Photo/File)
In this July 11, 2020, file photo, party members have their temperature checked and sanitize their hands as a precaution against the coronavirus at the national congress of the ruling Chama cha Mapinduzi (CCM) party in Dodoma, Tanzania. Tanzania's President John Magufuli openly expressed doubt about COVID-19 vaccines and accused people who were vaccinated outside the East African nation of bringing new infections into the country. (AP Photo/File) A security guard checks the temperature of a man wearing a face mask to protect against the spread of the coronavirus at a shopping and office complex in Beijing, Friday, Jan. 29, 2021. New cases of local transmission in China are continuing to fall with just several dozen announced on Friday, even as the country's annual Lunar New Year travel push gets underway. (AP Photo/Mark Schiefelbein)
A security guard checks the temperature of a man wearing a face mask to protect against the spread of the coronavirus at a shopping and office complex in Beijing, Friday, Jan. 29, 2021. New cases of local transmission in China are continuing to fall with just several dozen announced on Friday, even as the country's annual Lunar New Year travel push gets underway. (AP Photo/Mark Schiefelbein) Street vendors sell clothing and accessories for Baby Jesus figurines, as Mexicans prepare to celebrate "Dia de la Candelaria" or Candlemas Day amid a surge in the COVID-19 pandemic, in the center of Mexico City, Wednesday, Jan. 27, 2021. Although police and city officials are attempting to crack down on businesses and street vendors operating in defiance of coronavirus "red alert" restrictions, the merchants who rely on the seasonal Candlemas business say they have no choice but to work.(AP Photo/Rebecca Blackwell)
Street vendors sell clothing and accessories for Baby Jesus figurines, as Mexicans prepare to celebrate "Dia de la Candelaria" or Candlemas Day amid a surge in the COVID-19 pandemic, in the center of Mexico City, Wednesday, Jan. 27, 2021. Although police and city officials are attempting to crack down on businesses and street vendors operating in defiance of coronavirus "red alert" restrictions, the merchants who rely on the seasonal Candlemas business say they have no choice but to work.(AP Photo/Rebecca Blackwell) A man wearing a face mask to curb the spread of coronavirus walks past a mural by urban artist The Artful Dodger (A. Dee) of U.S. actor Chadwick Boseman who died last year of colon cancer aged 43, in Brixton, south London, Friday, Jan. 29, 2021, during England's third national lockdown since the coronavirus outbreak began. England is under an indefinite national lockdown to curb the spread of the new variant, with nonessential shops, gyms and hairdressers closed, most people working from home and schools largely offering remote learning. (AP Photo/Matt Dunham)
A man wearing a face mask to curb the spread of coronavirus walks past a mural by urban artist The Artful Dodger (A. Dee) of U.S. actor Chadwick Boseman who died last year of colon cancer aged 43, in Brixton, south London, Friday, Jan. 29, 2021, during England's third national lockdown since the coronavirus outbreak began. England is under an indefinite national lockdown to curb the spread of the new variant, with nonessential shops, gyms and hairdressers closed, most people working from home and schools largely offering remote learning. (AP Photo/Matt Dunham) Social distancing signs to try to curb the spread of coronavirus are displayed in the window of a temporarily closed shop in Brixton, south London, Friday, Jan. 29, 2021, during England's third national lockdown since the coronavirus outbreak began. England is under an indefinite national lockdown to curb the spread of the new variant, with nonessential shops, gyms and hairdressers closed, most people working from home and schools largely offering remote learning. (AP Photo/Matt Dunham)
Social distancing signs to try to curb the spread of coronavirus are displayed in the window of a temporarily closed shop in Brixton, south London, Friday, Jan. 29, 2021, during England's third national lockdown since the coronavirus outbreak began. England is under an indefinite national lockdown to curb the spread of the new variant, with nonessential shops, gyms and hairdressers closed, most people working from home and schools largely offering remote learning. (AP Photo/Matt Dunham) A Sri Lankan nurse administers a COVID-19 vaccine to an army soldier in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)
A Sri Lankan nurse administers a COVID-19 vaccine to an army soldier in Colombo, Sri Lanka, Friday, Jan. 29, 2021. Sri Lanka has begun inoculating frontline health workers, military troops and police officers against COVID-19 amid warnings about infections among medical workers. The island nation has received 500,000 doses of the AstraZeneca-Oxford University vaccine donated by India and manufactured by the Serum Institute of India. (AP Photo/Eranga Jayawardena)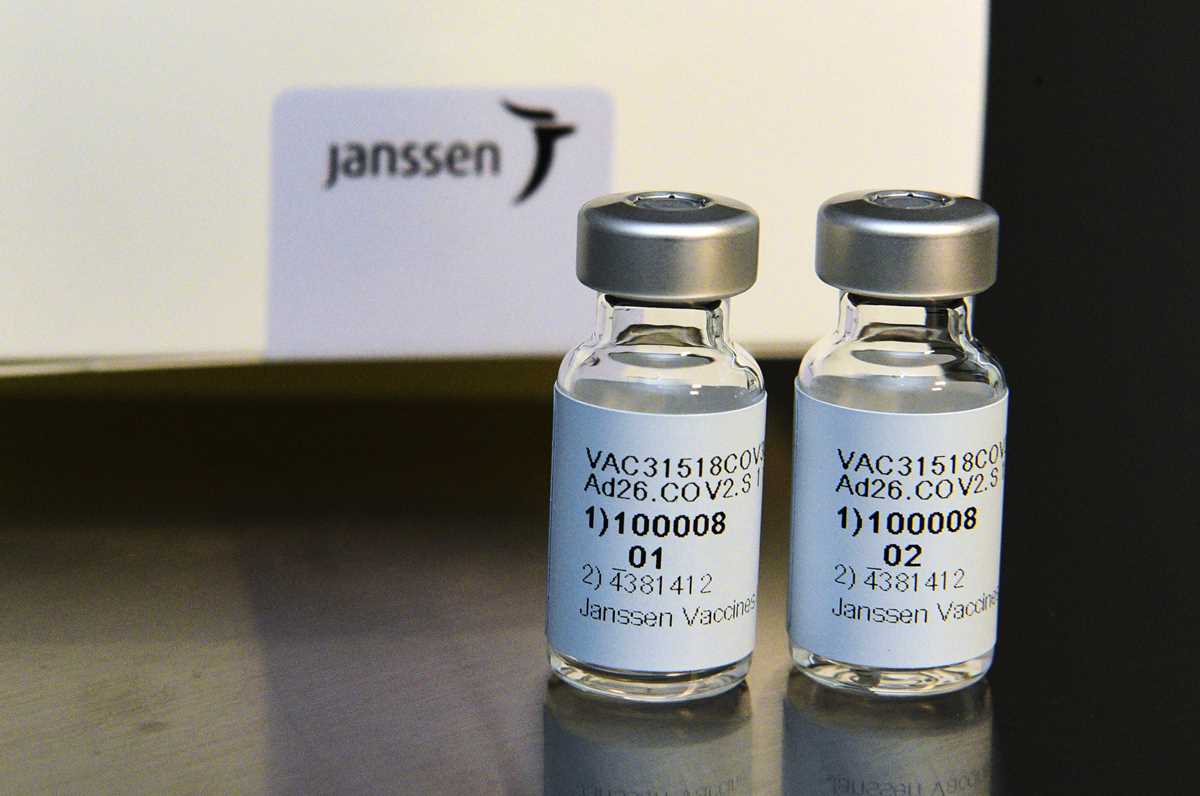 This Sept. 2020 photo provided by Johnson & Johnson shows the investigational Janssen COVID-19 vaccine. Johnson & Johnson's long-awaited COVID-19 vaccine appears to protect against symptomatic illness with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. Johnson & Johnson said Friday, Jan. 29, 2021 that in the U.S. and seven other countries, the first single-shot vaccine appears 66% effective overall at preventing moderate to severe COVID-19. It was more protective against severe symptoms, 85%. (Cheryl Gerber/Johnson & Johnson via AP)
This Sept. 2020 photo provided by Johnson & Johnson shows the investigational Janssen COVID-19 vaccine. Johnson & Johnson's long-awaited COVID-19 vaccine appears to protect against symptomatic illness with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. Johnson & Johnson said Friday, Jan. 29, 2021 that in the U.S. and seven other countries, the first single-shot vaccine appears 66% effective overall at preventing moderate to severe COVID-19. It was more protective against severe symptoms, 85%. (Cheryl Gerber/Johnson & Johnson via AP)WASHINGTON — The head of the CDC says doctors and public health officials should approach each new case of the coronavirus as if it is a mutation.
“I do believe we should be treating every case as it if it’s a variant during this pandemic right now,” Dr. Rochelle Walensky said during a White House coronavirus briefing.
Two cases of the variant that originated in South Africa has been detected in South Carolina. There is concern if it continues to spread, it could become dominant in a few months.
Walensky says contact tracing efforts in the U.S. are not yet up to the task of containing the potential breakout of new mutations.
White House coronavirus adviser Andy Slavitt says Congress must move quickly to pass President Joe Biden’s coronavirus relief bill, which contains money to expand efforts to track and identify mutations.
___
THE VIRUS OUTBREAK:
Johnson & Johnson 1-dose shot prevents virus, but less than some others. WHO team visits Wuhan hospital that had early coronavirus patients. The EU publishes vaccine contract, price redacted. Dubai blamed by several countries for spreading the coronavirus abroad after the city welcomed New Year’s revelers. Tanzania’s president says God has eliminated COVID-19 in the country; his own church begs to differ. Philadelphia's problematic vaccine rollout raises larger questions.
___
Follow all of AP’s pandemic coverage at https://apnews.com/hub/coronavirus-pandemic, https://apnews.com/hub/coronavirus-vaccine and https://apnews.com/UnderstandingtheOutbreak
___
HERE’S WHAT ELSE IS HAPPENING:
WASHINGTON — Dr. Anthony Fauci says the emergence and increasing spread of coronavirus mutations means vaccine makers must be ready to make new shots to stay ahead of the public health crisis.
The government’s top infectious disease expert spoke Friday during a White House coronavirus briefing.
“This is a wake-up call to all of us,” says Fauci, noting government scientists will be working to keep pace with virus mutations.
The nature of viruses is to change in ways that promote their spread, Fauci says. The evolution of mutant versions means scientists need to be “nimble” and ready to make tweaks to vaccines. So far, the mutants haven’t overwhelmed the protective power of vaccines.
Fauci says it is important to vaccinate people as quickly as possible to keep new mutations from developing.
___
BERLIN -- Regulators have authorized AstraZeneca’s coronavirus vaccine for use in adults throughout the European Union, amid criticism the bloc is not moving fast enough to vaccinate its population.
The European Medicines Agency licensed the vaccine Friday to be used in people 18 and over, although concerns had been raised this week that not enough data exist to prove it works in older people.
The shot is the third COVID-19 vaccine given the greenlight by the European Medicines Agency, after those made by Pfizer and Moderna. Both were authorized for all adults.
___
WASHINGTON — The Biden Administration won’t need any new coronavirus vaccines approved by the FDA to fulfill its plan to purchase 200 million additional doses by the summer.
White House press secretary Jen Psaki says while it’s a “positive step” for additional vaccines to receive FDA approval, the Biden Administration will rely solely on Moderna and Pfizer for its new purchases.
Johnson & Johnson announced Friday its single-dose coronavirus vaccine was effective, though at a lower rate than the Moderna and Pfizer vaccines. The company is seeking emergency-use authorization from the FDA.
Psaki says the Biden administration would “rely on our health and medical experts to advise” on additional vaccines.
___
TOKYO — Japanese Prime Minister Yoshihide Suga says he is determined to host the postponed Tokyo Olympics this summer, despite growing uncertainty as coronavirus cases rise at home.
Suga, speaking at a virtual meeting of the World Economic Forum, says the Olympics would be a symbol of human victory over the pandemic. He pledged to get infections under control in Japan as soon as possible and achieve a “safe and secure” Olympics.
Olympic officials have repeatedly said the games will be held in July as planned after a one-year postponement, though various scenarios including the holding of events without spectators are being considered.
Suga has been criticized for delaying virus measures until daily cases surged to new highs in late December. He eventually declared a partial state of emergency in early January, issuing non-binding requests through Feb. 7 for people to avoid crowds or eating out in groups and for restaurants and bars to close early.
New coronavirus cases in Tokyo have dipped but experts say they haven’t slowed enough, indicating the emergency measures could be extended for several more weeks.
___
KYIV, Ukraine — The parliament of Ukraine has passed a law banning registration of the coronavirus vaccine developed by Russia.
The vaccine, called Sputnik V, has been registered for use in 11 countries as well as in Russia. The parliament on Friday approved registration for vaccines used in the United States, China, Japan and the European Union, among other countries, but specifically excluded vaccines developed by “the aggressor state.”
Ukraine’s relations with Russia plunged after the 2014 Russian annexation of Crimea and the continuing conflict in the country’s east with Russia-backed separatists. Ukraine’s underfunded medical system has been hit hard by the coronavirus. The country of 44 million has recorded more than 1.2 million infections and more than 22,000 confirmed deaths.
___
NEW BRUNSWICK, N.J. — Johnson & Johnson says its vaccine appears to protect against COVID-19 with just one shot.
It’s not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. Results released Friday show the single-shot vaccine was 66% effective overall at preventing moderate to severe illness, and much more protective against the most serious symptoms.
These are preliminary findings from a study of 44,000 volunteers that is ongoing. Researchers tracked illnesses starting 28 days after vaccination – about the time when, if participants were getting a two-dose variety instead, they would have needed another shot.
After day 28, no one who got vaccinated needed hospitalization or died regardless of whether they were exposed to “regular COVID or these particularly nasty variants,” Dr. Mathai Mammen, global research chief for J&J’s Janssen Pharmaceutical unit, told The Associated Press. When the vaccinated did become infected, they had a milder illness.
The vaccine worked better in the U.S. compared to South Africa, where it was up against a tougher, mutated virus. The company says it will file an application for emergency use soon in the U.S., and then abroad.
It expects to have some ready to ship as soon as authorities give the green light and supply 100 million doses to the U.S. by June.
___
BERLIN — Swiss pharmaceutical company Novartis says it has signed an agreement to help production of the Pfizer-BioNTech coronavirus vaccine.
Novartis says the initial agreement calls for it to take active ingredient from Germany’s BioNTech and fill it into vials at its plant in Stein, Switzerland. The vials would be sent back to BioNTech for distribution.
Novartis says the plan is for it to start production during the second quarter, with the initial shipment of finished vaccines expected in the third quarter.
Novartis’ announcement comes after another rival, French drugmaker Sanofi, said Wednesday it would help bottle and package the BioNTech-Pfizer vaccine at a plant in Frankfurt.
___
PARIS — Nearly two weeks after France extended its vaccine campaign to over-75-year-olds, elderly residents across Paris are flocking to vaccination centers amid concerns of a vaccine shortfall.
France is expecting less than a third of planned deliveries of the AstraZeneca vaccine this quarter – 4.6 million doses instead of 15.8 million, according to French officials.
Nurses at a medical center in Paris’ residential 15th district are currently administering between 80 and 100 shots a day – a number that could drop.
“Our goal here is to administer the second jab within the recommended timeframe,” said the center’s Chief Doctor François Teboul. “The likely consequence is we will have to push back a number of first vaccinations in the weeks to come.”
Delays or production problems for the Pfizer-BioNTech vaccine have caused concerns across the European Union.
___
WASHINGTON — The new director of the Centers for Disease Control says officials have “scaled up” their surveillance of new coronavirus variants in the United States.
CDC Director Rochelle Walensky told ABC’s “Good Morning America” that previously “there has not been a public health infrastructure” to track such variants. Also, there weren’t resources to do “mass sequencing” of the virus across the country. She noted the coronavirus aid plan pushed by the Biden Administration includes funds to improve such tracing.
However, Walensky says it was “concerning” the two South Carolina individuals who were diagnosed with the more virulent strain first identified in South Africa didn’t know each other or travel there, so the “presumption” is there’s “community spread of this strain.”
___
BRUSSELS — The European Union has made public a redacted version of the contract it agreed with the drugmaker AstraZeneca which lies at the heart of a major row over coronavirus vaccine deliveries.
The EU’s executive branch, the European Commission, published the text of the advance purchasing agreement Friday after consulting the British-Swedish company.
Details about the price of the vaccine were notably redacted. The U.K. is thought to be paying far more for the vaccine than the 27-nation bloc.
Earlier this week, the EU lashed out at the drugmaker after it said it would not be able to deliver the 80 million doses that it hoped to provide and could only supply 31 million.
The advance purchasing agreement was signed before any vaccine existed. European authorities are expected to approve the AstraZeneca vaccine later Friday, but questions remain over how effective it is among people over 65.
___
ROME — Italy’s virus czar says pharmaceutical company Moderna officially advised the government Friday that it would reduce a planned upcoming vaccine delivery to Italy by 20%, fueling increasing outrage in Italy as such delays have forced the country to drastically slow down its vaccine campaign.
Domenico Arcuri expressed “stupor, concern and discomfort” at Moderna’s decision, noting that it came after both Pfizer and AstraZeneca announced similar delays in scheduled deliveries. The Italian government has formally advised Pfizer it is weighing legal action.
Arcuri said Moderna told the government its Feb. 8 deliveries would be 132,000 doses instead of a planned 166,000.
The reduced deliveries have meant that Italy’s plan to start vaccinating Italians over age 80 on a mass scale have been delayed by several weeks, and reduced by more than half the number of shots administered each day. Italy concentrated the first weeks of its vaccine campaign on health care workers, with about 1 million of the 1.7 million doses administered so far going to doctors and nurses.
___
MADRID — Spain’s health minister says that between 5 and 10% of all confirmed coronavirus infections are believed to be derived from a mutation seen as responsible for the high contagion rates seen first in the United Kingdom and later in other countries.
Appearing Friday at a Congress of Deputies health commission, Carolina Darias said that so far confirmed cases of the new variant in Spain stand at 350 but that experts’ analysis showed that up to 10% of new infections could be attributed to it.
“The following weeks are crucial to see if this variant takes over ... like has happened in other countries,” Darias told lawmakers.
On Thursday Spain logged nearly 35,000 new cases of the virus and 515 confirmed deaths, although the 14-day rate of infection per 100,000 residents dropped slightly for the first time in nearly a month.
Authorities say that an abrupt decline would be needed for overwhelmed hospitals to free up space before any effects of the new variant increases contagion and hospitalizations again.
___
BELGRADE, Serbia — Serbia has received an additional 40,000 doses of the Sputnik V vaccine from Russia, further boosting its so far successful coronavirus vaccination campaign when compared with the rest of the Balkans.
While its neighbors have struggled to secure the shots, Serbia has embarked on one of the fastest vaccine rollouts in Europe with some 400,000 people getting jabs so far in the country of 7 million.
One million doses of Chinese Sinopharm vaccines arrived in Serbia earlier this month as well as thousands of doses of the Pfizer and Sputnik V shots.
Serbian officials say all the vaccine shipments which have arrived have been directly negotiated with either Russia and China, or with the Pfizer producers in the U.S. They say that so far no vaccines have been delivered by the global Covax system aimed at providing affordable shots to poor and middle-income countries.
Serbia is also negotiating the domestic production of the Russian Sputnik V vaccine. The Russian and Chinese vaccines have not been approved by the European Union drug regulators.
___
AMSTERDAM — The European Medicines Agency says no new side effects linked to the coronavirus vaccine made by BioNTech and Pfizer were identified in the regulator’s first safety update on COVID-19 vaccines.
In a statement published Friday, the European regulator said its expert committee assessed reports of people who died after getting the vaccine and said their review “did not suggest a safety concern.” Earlier this month, Norwegian officials amended their vaccination advice to say that doctors should assess frail and severely ill elderly people to decide if they should be immunized.
The EMA concluded that safety data collected on the Pfizer vaccine are “consistent with the known safety profile of the vaccine” and noted that severe allergic reactions are a known, rare side effect. It said the frequency of such allergic reactions was about 11 cases per million doses in the U.S. but that there was no comparable European estimate yet.
The EMA authorized the Pfizer vaccine on December 21 and granted it a conditional license; Pfizer and BioNTech must submit safety reports every month in line with a heightened monitoring process. The agency said “there are no recommended changes regarding the use of the vaccine.”
___
BUDAPEST, Hungary — Hungary’s prime minister says a deal to purchase Chinese coronavirus vaccines could be concluded as early as Friday, which would make Hungary the first country in the European Union to purchase a vaccine from China.
Prime Minister Viktor Orban said on public radio that his government is monitoring the use of a vaccine developed by Chinese state-owned company Sinopharm in neighboring Serbia, which became the first European country to administer the drug after it received 1 million doses earlier this month.
Hungary’s drug regulator has not yet approved the Sinopharm vaccine. But a decree ordered by the government on Thursday streamlined the approval procedure by allowing any vaccine that had been administered to at least 1 million people to be used in Hungary.
Orban said he would personally choose to be inoculated with the Sinopharm vaccine since he trusted it the most.
Hungary last week was the first EU member to approve the Russian Sputnik V vaccine, and has ordered doses to treat 1 million people over the next three months. Orban has been critical of the EU’s vaccine procurement program and insisted that countries that pursue their own vaccine agreements are faring better.
___
Before you consider Johnson & Johnson, you'll want to hear this.
MarketBeat keeps track of Wall Street's top-rated and best performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat has identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market catches on... and Johnson & Johnson wasn't on the list.
While Johnson & Johnson currently has a "Hold" rating among analysts, top-rated analysts believe these five stocks are better buys.
View The Five Stocks Here
Need to stretch out your 401K or Roth IRA plan? Use these time-tested investing strategies to grow the monthly retirement income that your stock portfolio generates.
Get This Free Report